
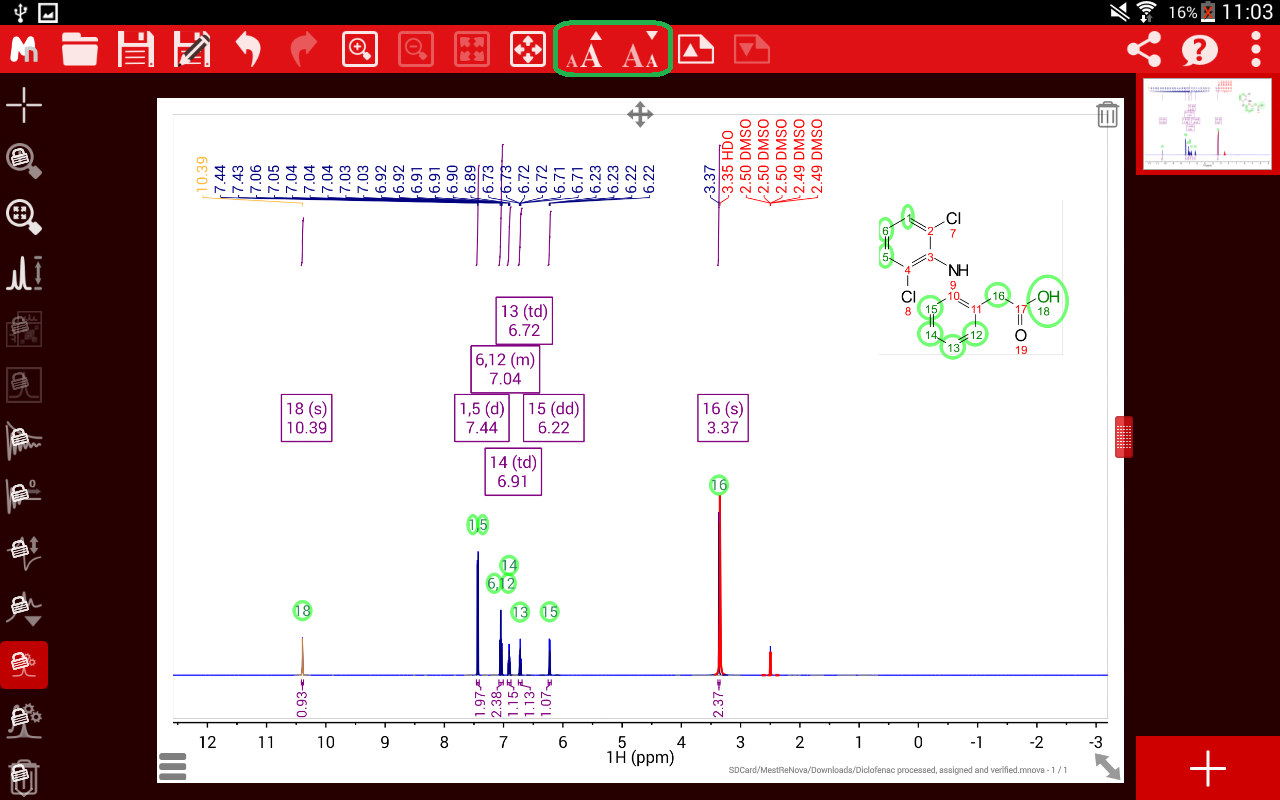
The measurement of two-bond proton-proton coupling constants (2JHH) in prochiral CH2 groups from the F2 dimension of 2D spectra is not easy due to the usual presence of complex multiplet J patterns, line broadening effects and strong coupling artifacts. The last remaining peak at 4.999 ppm must be proton 13 this is confirmed by COSY correlation with proton 12, triplet multiplicity based on splitting by proton 12, and integration of one proton.2J(HH)-resolved HSQC: Exclusive determination of geminal proton-proton coupling constants We can assign proton 12 (3.564 ppm) based on its integration of 2H and its COSY correlation to proton 11. Proton 7’s peak at 6.163 ppm is split into a triplet by the two 8 protons, confirming the assignment.Īll that remains are protons 12 and 13. Once proton 8 has been assigned, we can easily assign proton 7 based on the remaining COSY correlation for proton 8. Therefore, we can assign proton 10 as 5.209 ppm and proton 11 as 3.754 ppm.

To differentiate protons 10 and 11, take a look at our COSY table 3.754 ppm shows two COSY correlations, while 5.209 ppm only shows one. From this list, we can easily assign proton 8 as the peak at 2.068 ppm based on its integration of 2 protons. Based on the COSY, proton 9 couples protons at 2.068 ppm (2H), 3.754 ppm (1H), and 5.209 ppm (1H). Thymidine’s structure suggests that proton 9 should couple protons 8, 10, and 11. Now that proton 9 has been assigned, the fun really begins. The remaining protons are doublets, triplets, and multiplets that can be assigned by 2-dimensional COSY. The peak also integrates to 1 proton, supporting the assignment. The chemical shift of 11.256 ppm supports this assignment, as imide protons often show up far downfield. The only proton that should show up as a singlet is proton 6, as it has no neighboring protons that would split the peak (the nearest proton is 5 bonds away!). There is only one singlet in the ¹H-NMR spectrum. Therefore, the peak at 7.690 ppm must represent proton 4! The integration and chemical shift support the assignment, as proton 4 is the only aromatic proton in the structure. The long-range coupling constant observed for proton 3 (J=1.2 Hz, split into a doublet by proton 4) is reflected in the coupling constant for proton 4 (J=1.2 Hz, split into a quartet by proton 3). Protons that are coupled to each other should exhibit the same coupling constant. The peak is split into a doublet with a coupling constant of 1.2 Hz, reflecting the long-range coupling between protons 3 and 4, which also supports this assignment.
The high field chemical shift supports this assignment. The only peak with an integration of 3 is the doublet at 1.770 ppm. Proton 3 is the only methyl group in the structure, and therefore must integrate to 3 protons.


 0 kommentar(er)
0 kommentar(er)
